Before diving into what a semi-solid state battery is, let’s first take a look at how a traditional lithium-ion battery works. Fundamentally, such a battery consists of four key components: the cathode (positive side), the anode (negative side), the electrolyte (the conductor between positive and negative), and a separator.A LiFePO4 battery, also known as LFP or lithium iron phosphate, contains the following materials:
- Cathode: Made of lithium iron phosphate (LiFePO4). This material gives the battery its name and is known for its stability and safety.
- Anode: Typically made of graphite, which provides a storage area for lithium ions during charging cycles.
- Electrolyte: Consists of organic solvents with a lithium salt, such as lithium hexafluorophosphate (LiPF6), enabling ion transport between the cathode and anode.
- Separator: A permeable membrane that prevents direct electrical contact between the cathode and anode while allowing ions to pass through the electrolyte. This ensures electrons flow through the external circuit.
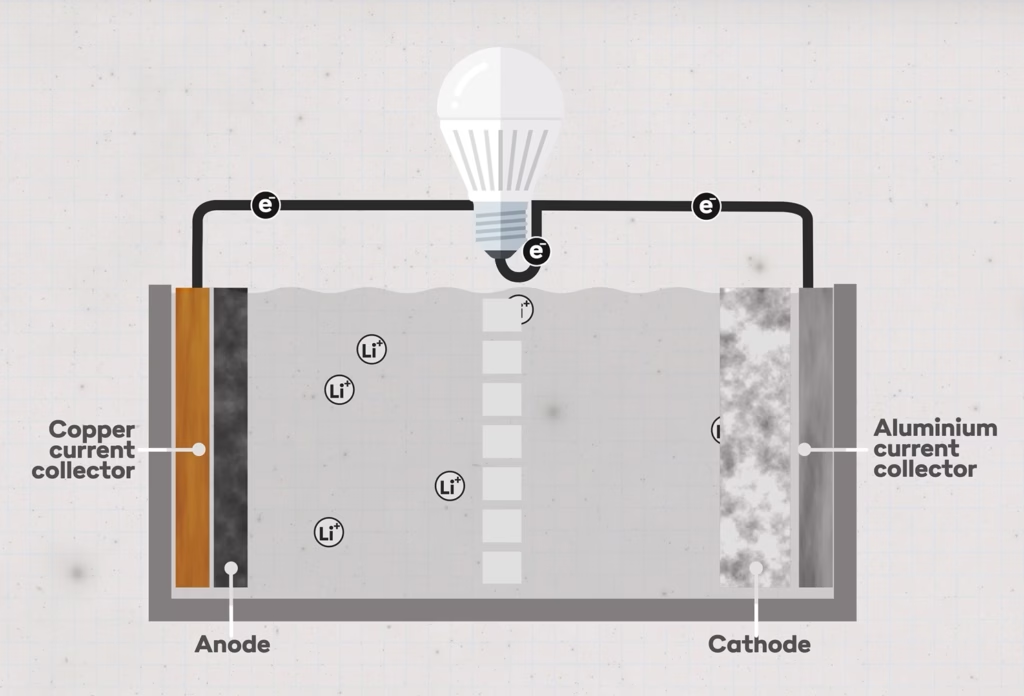
(Image source: Dave Borlace – Just Have a Think)
Semi-solid state technology combines elements from both traditional and solid-state batteries. This technology uses a partially solid electrolyte, offering improved safety compared to liquid electrolytes. Semi-solid state batteries have higher energy density and perform better in extreme temperatures. Looking at the components of semi-solid state batteries, cathode, anode, and electrolyte (but no separate separator), we can highlight the following:
- Cathode: Still made of lithium iron phosphate (LiFePO4) but can be integrated with a solid electrolyte to enhance stability and safety.
- Anode: Typically still uses graphite, though there is potential for other materials that can integrate with solid electrolytes to increase energy density and cycle life.
- Electrolyte: Replaces the liquid electrolyte with a solid or semi-solid one, which may be a polymer or another solid substance. This reduces leakage risks and improves safety.
- Separator: In semi-solid state batteries, the separator may be part of the solid electrolyte, reducing the need for a separate membrane structure and enabling a more compact, safer design.
These advancements aim to enhance safety, reduce fire risks, and improve battery lifespan and energy density. By using a lower volume of semi-solid electrolyte, there is less flammable material and a reduced risk of short circuits due to the higher viscosity (thicker consistency) of the electrolyte.
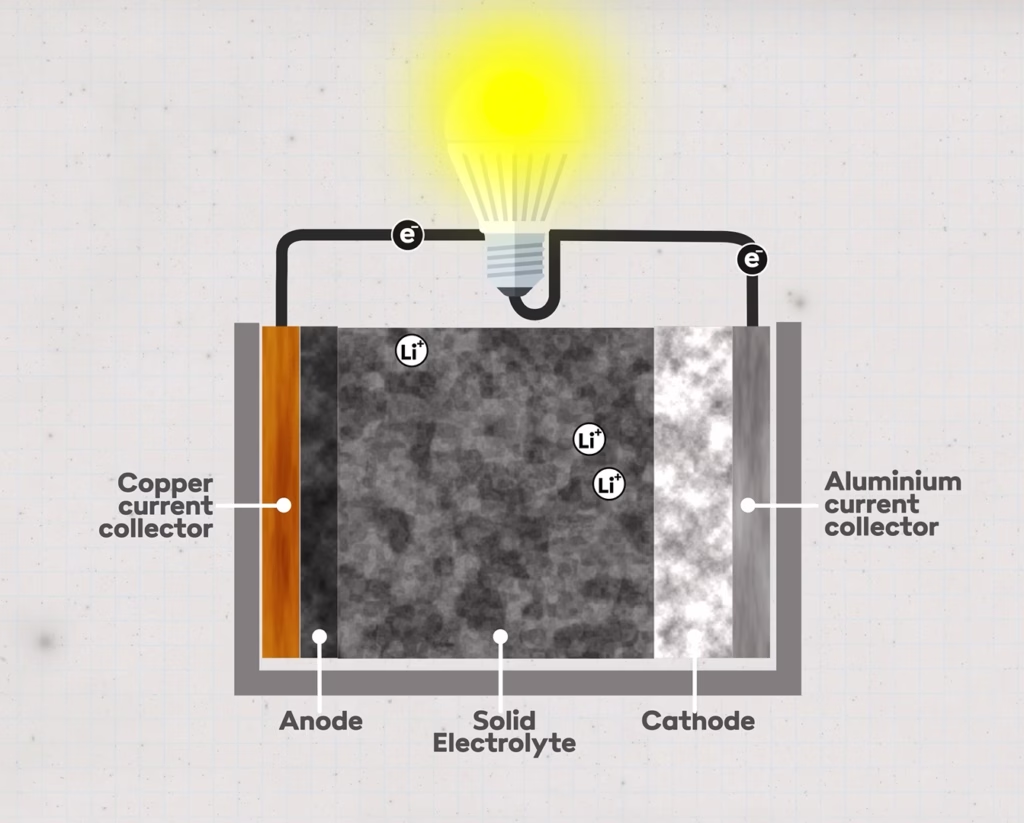
(Image source: Dave Borlace – Just Have a Think)
Want to learn more about semi-solid state technology? Check out a video by YouTuber Dave Borlace on his channel, “Just Have a Think.” In the video, he gives a detailed overview of how the technology works, sharing both its advantages and challenges: Would you be OK with a Semi Solid State?



